Hyperlipidaemia
Notes
Overview
Hyperlipidaemia broadly refers to having too many lipids (i.e. fats) in the blood.
Hyperlipidaemia is a broad term that refers to high levels of one or more of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG). Hyperlipidaemia is very common and a major risk factor in the development of atherosclerosis and cardiovascular disease. Importantly, high levels of high-density lipoprotein cholesterol (HDL-C), commonly referred to as ‘good’ cholesterol, actually confers cardiovascular protection.
Various terms for excess serum lipids including hypercholesterolaemia, dyslipidaemia and hyperlipidaemia are often used interchangeably in clinical practice but refer to specific problems with lipid levels and metabolism.
- Hypercholesterolaemia: an increase in TC and/or LDL-C. Commonly used synonymously with hyperlipidaemia
- Dyslipidaemia: a wider term that also includes low levels of HDL-C
- Hypertriglyceridaemia: an excess amount of triglycerides
- Familial hyperlipidaemia: excess serum lipids due to a genetic defect in lipid metabolism. Various subtypes
For more information on the genetic causes of hyperlipidaemia, check out our Familial hyperlipidaemia note.
Epidemiology
The prevalence of hyperlipidaemia is dramatically increasing in line with the rising rates of obesity.
Without medical and dietary intervention, hyperlipidaemia is a chronic and progressive disease that increases the risk of cardiovascular disease. Worryingly, the UK has one of the highest average cholesterol levels in the world.
Hyperlipidaemia is a major cause of premature coronary artery disease (CAD) and we known that TC is an important predictor of cardiovascular events. Obesity is a major factor in the development of hyperlipidaemia. Patients with obesity typically have high TG, high LDL-C and decreased HDL-C. In addition, there is a significant underdiagnosis of familial hyperlipidaemia.
Aetiology
Hyperlipidaemia may be considered primary or secondary.
Many factors contribute to the development of hyperlipidaemia. These may be due to one or more underlying genetic mutations known as ‘primary hyperlipidaemia’ or due to some underlying disease or environmental factors known as ‘secondary hyperlipidaemia’.
Primary (familial)
Primary, or familial, causes of hyperlipidaemia refer to the inheritance of an abnormal gene involved in lipid metabolism that increases the level of certain lipids in the blood (e.g. cholesterol, triglycerides, or lipoproteins). This can lead to significant problems including early-onset CAD.
There are different subtypes of primary hyperlipidaemia, which depend on the clinical picture of elevated lipids and underlying genetic mutation. Some conditions may be due to a single abnormal gene (i.e. monogenic), whereas others may be due to multiple genetic mutations (i.e. polygenic) that collectively contribute to the elevation of lipids.
The more frequently recognised primary hyperlipidaemias are mentioned below:
- Familial hypercholesterolaemia (FH): most severe type. Estimated prevalence is 1 in 500 of the general population. Marked hypercholesterolaemia. Characterised by significantly elevated LDL-C
- Common (polygenic) hypercholesterolaemia: a common disorder of LDL metabolism. Genetics poorly understood with multiple genes contributing to elevated LDL-C
- Familial combined hyperlipidaemia: common disorder seen in 1-2% of the population. Characterised by elevations in TC, TG and LDL-C. Generally considered a complex polygenic condition
- Polygenic hypertriglyceridaemia: leads to mild-to-moderate elevations in triglycerides with multiple contributing abnormal genes
- Chylomicronaemia syndrome: a variety of polygenic and monogenic conditions that result in significantly elevated triglycerides. A fasting blood sample shows lipid particles floating on top (especially after leaving the sample overnight)
For more information see our Familial hyperlipidaemia note.
Secondary (acquired)
Hyperlipidaemia due to a secondary cause is common in the population. Conditions most commonly implicated in hyperlipidaemia are excess alcohol intake and diabetes mellitus. The reason behind hyperlipidaemia is complex and the pattern of abnormal lipids depends on the underlying cause.
Major secondary causes include:
- Diabetes mellitus
- Obesity
- Nephrotic syndrome
- Smoking
- Excess alcohol
- Medications (e.g. thiazide diuretics, olanzapine, proteas inhibitors)
- Chronic kidney disease
- Hypothyroidism
- Cholestatic liver disease
Lipid metabolism
Cholesterol and triglycerides are essential within the body for normal cellular functions.
Lipids such as cholesterol and triglycerides may be absorbed from our diet by the intestines and then transported around the body after modifications. Alternatively, cholesterol may be synthesised de novo by different organs. In the liver, cholesterol is synthesised and packaged into lipoprotein structures that are then transported to peripheral tissues. These two pathways are known as the exogenous and endogenous lipid pathways.
Exogenous pathway
In this pathway, dietary lipids are broken down in the gut by pancreatic lipase. The lipids are metabolised into free fatty acids (FFA) and monoacylglycerol. These are then emulsified by bile acids to form micelles and taken up by enterocytes where they reform into triglycerides. Within enterocytes, cholesterol and triglycerides are packaged into chylomicrons. These are large triglyceride-rich particles that are needed for the transport of dietary triglycerides and cholesterol to peripheral tissue. They contain several apolipoproteins (molecules that bind lipids to form lipoproteins), with Apo B-48 being the core structural protein.
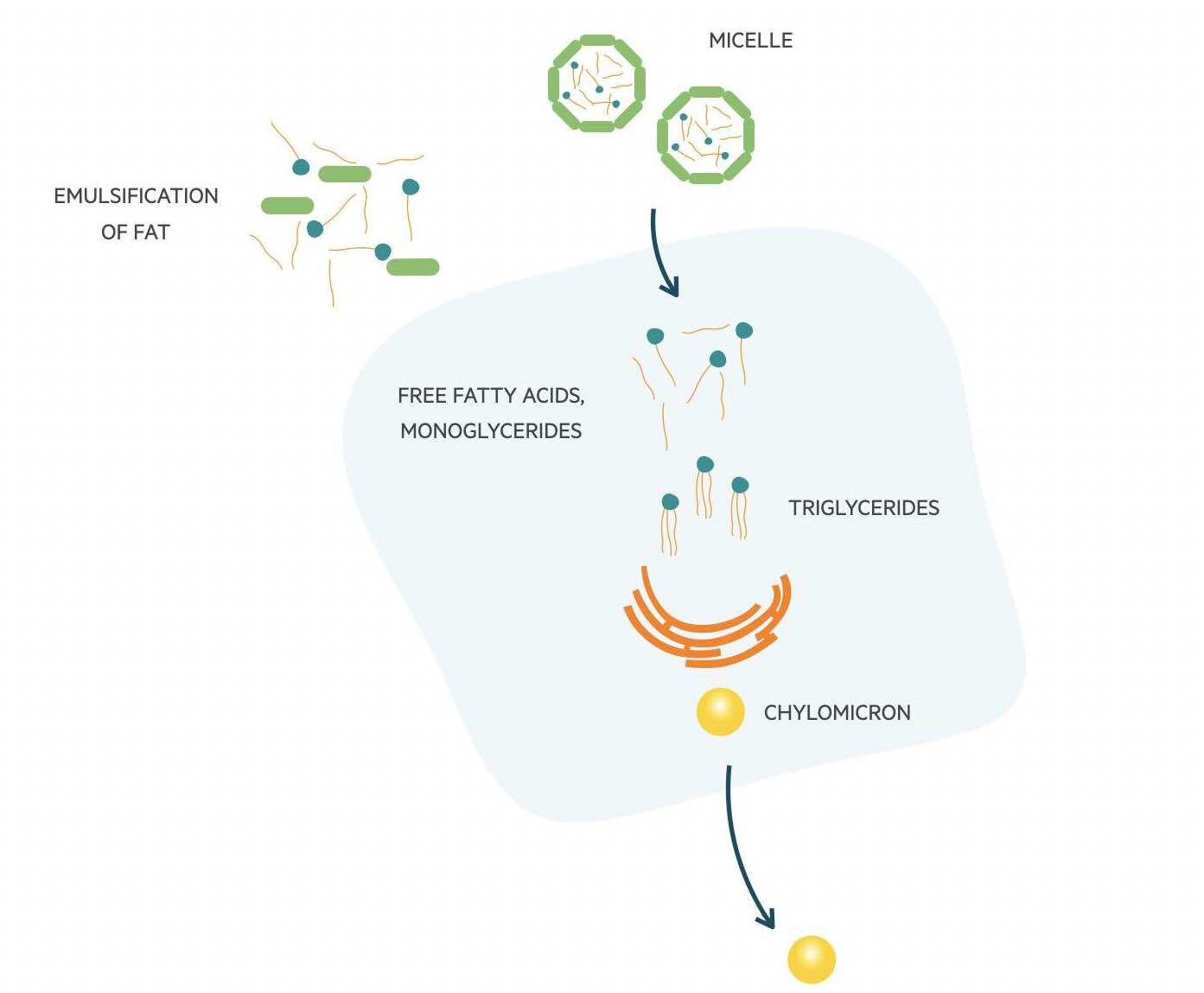
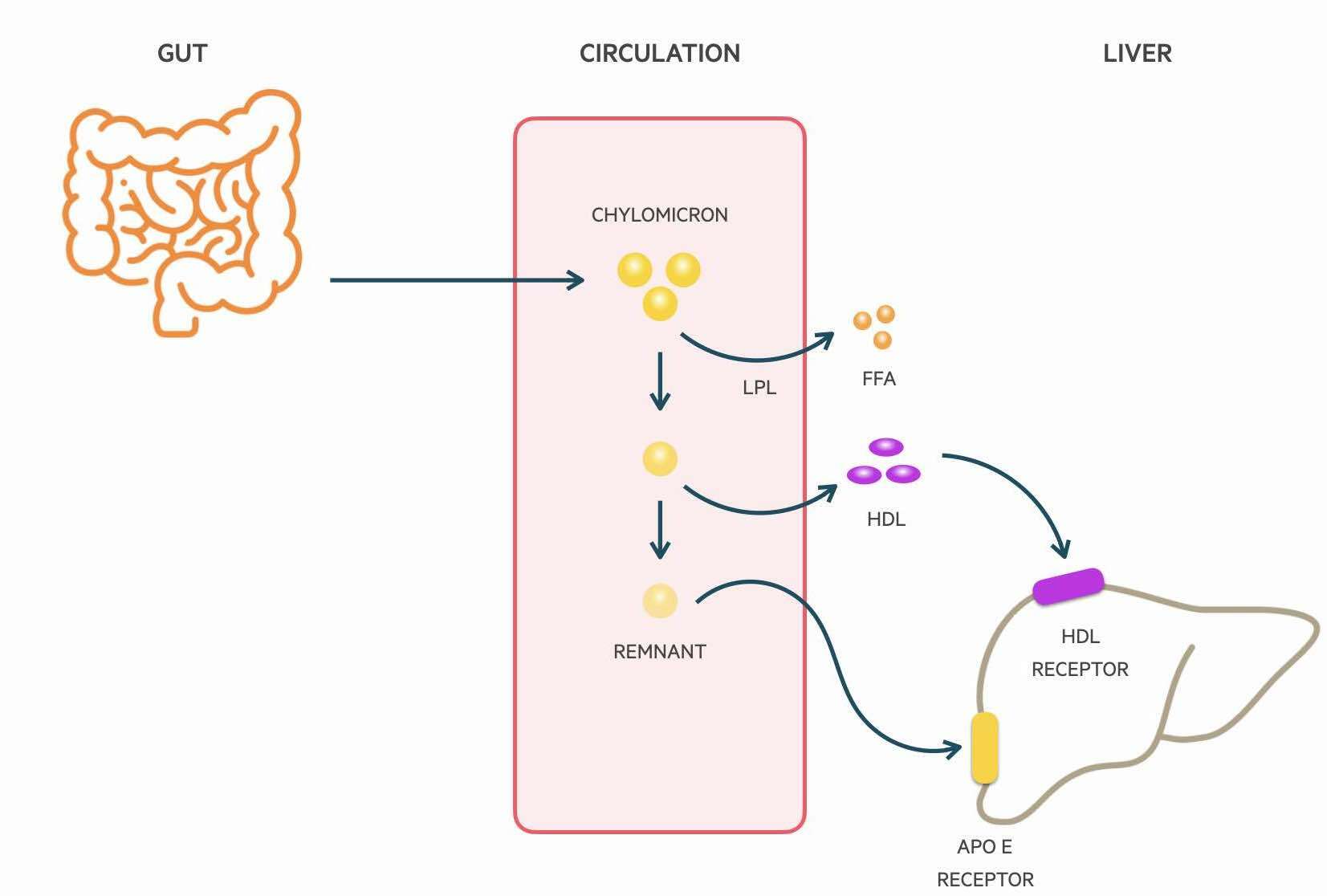
Chylomicrons are absorbed through the lymphatic system and delivered to the systemic circulation via the thoracic duct. Lipoprotein lipase (LPL) located on the luminal surface of capillaries is able to hydrolyse triglycerides which releases FFAs for uptake by muscle cells and adipocytes. The metabolism of triglycerides leads to a decrease in the size of chylomicrons and the formation of chylomicron remnants. Phospholipids and apolipoproteins are transferred to other lipoproteins such as HDL-C. The remnants are finally cleared from the circulation by the liver. It is Apolipoprotein E on the chylomicron that is able to bind to LDL receptors and be uptake by hepatocytes.
Endogenous pathway
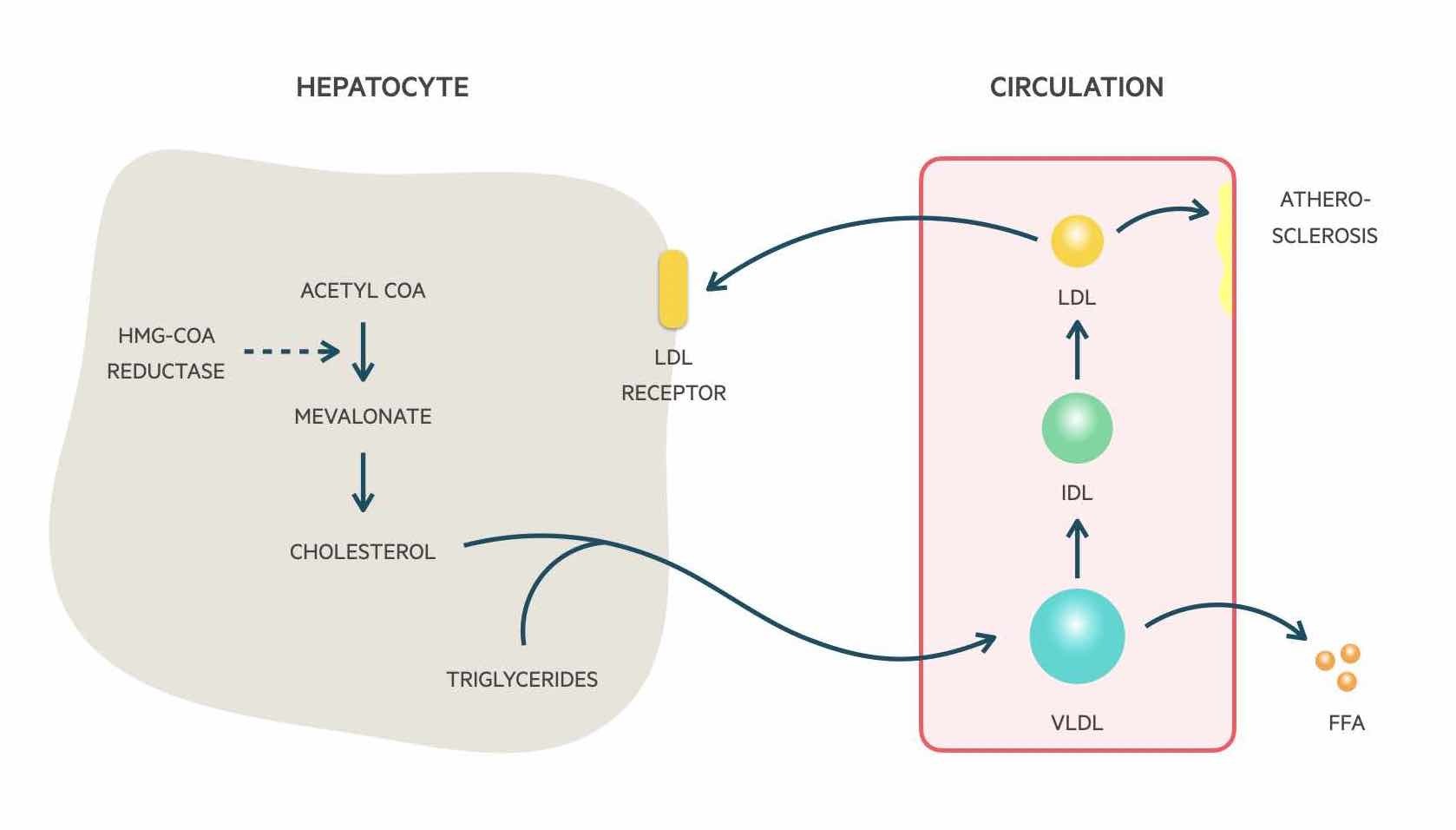
In the liver, cholesterol may be synthesised de novo by the conversion of acetyl coenzyme A into mevalonate and then cholesterol by the action of HMG-CoA reductase, which is the enzymatic target of statins. Cholesterol and triglycerides are formed into very-low-density lipoproteins (VLDL). They are triglyceride-rich and the core structural protein is Apo B-100.
VLDLs are secreted into the circulation. LPL found in peripheral tissues then hydrolyse triglycerides found in these molecules to enable the release and uptake of fatty acids into cells similar to that of chylomicron metabolism. Removal of triglycerides leads to the formation of Intermediate density lipoproteins (IDL) that are cholesterol-rich and obtain Apo E from HDL-C particles. These molecules may be taken up by the liver or further metabolised to form low-density lipoproteins (LDL).
The majority of LDLs are cleared from the circulation by LDL receptors found on hepatocytes. The remainder is cleared by peripheral tissue. The more LDL receptors there are on hepatocytes, the more clearance of LDL from the circulation. This process is regulated by the cholesterol content of hepatocytes. This whole process enables the transport of triglycerides and cholesterol formed in the liver to peripheral tissue including muscle and adipose tissue.
Pathophysiology
Hyperlipidaemia can lead to the early development of coronary artery disease.
Abnormally high lipids are a major risk factor for the development of ischaemic heart disease through the process of atherosclerosis. This describes the deposition of fatty material on the inner lining of arteries. This leads to narrowing, ischaemia and if the plaque ruptures, sudden occlusion. Multiple factors contribute to the development of atherosclerosis, not just hyperlipidaemia. These include endothelial damage, inflammation and immunologic factors.
Major lipid abnormalities that increase the risk of cardiovascular disease include:
- High TC
- Low HDL-C: inversely correlated with cardiovascular events
- High TG: especially in the context of low HDL-C
- High non-HDL-C: the difference between TC and HDL-C
The majority of cases of hyperlipidaemia are multifactorial, resulting from multiple secondary factors (e.g. obesity, smoking, alcohol) and underlying hereditary predisposition. If the genes predisposing to hyperlipidaemia result in a significant impact on serum lipids levels they will cause primary hyperlipidaemia.
Clinical features
Clinical features may only be seen in patients with very high levels of lipids.
Hyperlipidaemia is usually an incidental finding on screening for lipid abnormalities in at-risk patients (e.g. cardiac event, family history, chronic kidney disease). In patients with very high lipid levels, which is often due to primary hyperlipidaemia, there may be clinical signs of lipid deposition in tissue.
Signs to look out for:
- Corneal arcus: white or grey opaque ring in the corneal margin
- Tendon xanthoma: hard, non-tender nodular enlargement of tendons. Common on knuckles and achilles
- Xanthelasma: yellow plaques on the upper eyelids
Diagnosis & investigations
A full lipid profile is vital to assess patients' risk of cardiovascular disease and response to anti-lipid therapy.
A diagnosis of hyperlipidaemia is based on a full lipid profile. This is a blood test that provides all the key levels of serum lipids. If the levels are very high, it may indicate underlying primary hyperlipidaemia. In these cases, there are specific referral criteria (see below).
Levels above the upper limit of normal are considered abnormal. Serum lipid levels are used in the context of other factors in the patients' history to determine the need for treatment.
- Established cardiovascular disease: anti-lipid therapy offered. Response to serum lipid assessed following treatment
- No established cardiovascular disease: serum lipids are used as part of a cardiovascular risk score (QRISK2) to determine the need for anti-lipid therapy
Lipid profile
This is a simple blood test that should be completed as a non-fasting test. The test provides the following results:
- Serum cholesterol (2.5 - 5.0 mmol/L)
- Serum LDL cholesterol (0 - 3.5 mmol/L)
- Serum HDL cholesterol (0.9 - 1.5 mmol/L)
- Serum triglycerides (0.4 - 2.3 mmol/L)
- Non-HDL cholesterol (serum cholesterol - HDL cholesterol)
- Serum cholesterol/HDL ratio
If serum triglycerides are > 4.5 mmol/L, then a repeat fasting sample is recommended.
QRISK
This is a risk calculator that is freely available online. It is a tool that incorporates different bits of information in the patients' history (e.g. BMI, renal function, serum lipids, smoking status) to determine their risk of having a cardiovascular event (e.g. heart attack, stroke) over the next ten years. There are different versions of the calculator with the QRISK2 currently recommended for assessing cardiovascular risk.
Patients with a QRISK ≥10% over the next ten years, even in the absence of established cardiovascular disease, should be offered anti-lipid therapy. In patients with, or suspected to have, primary hyperlipidaemia the tool should not be used.
NOTE: the QRISK may underestimate risk in certain groups (e.g. patients with HIV, those taking antipsychotics, severe obesity, those already on cardiovascular medications). This should be taken into consideration when deciding on treatment.
Initial investigations
When determining hyperlipidaemia as part of cardiovascular risk assessment, it is important to complete a series of initial investigations, especially before any treatment. These include:
- Non-fasting full lipid profile
- HbA1c
- Assess for secondary causes of hyperlipidaemia (e.g. alcohol, smoking, CKD)
- Assess risk for anti-lipid therapy (includes baseline renal, thyroid, and liver profiles +/- CK if unexplained muscle pain)
- Determine any contraindications/drug interactions
- If non-fasting TG > 4.5 mmol/L arrange a fasting sample
Referral (suspected familial)
Patients with, or suspected to have, primary hyperlipidaemia should be referred to a specialist lipid clinic.
When determining a patients' risk of familial hyperlipidaemia it is important to consider the serum lipid levels, clinical features (e.g. any xanthelasma or xanthoma) and family history of lipid disorders or early cardiovascular disease.
The national institute of clinical excellence (NICE) produced guidelines for Familial hypercholesterolaemia: identification and management (CG71) in 2008 with updates in 2019. These coincide with their guidelines on Cardiovascular disease: risk assessment and reduction, including lipid modification (CG181) produced in 2008 with updates in 2016.
Suspecting familial hypercholesterolaemia
Familial hypercholesterolaemia should be suspected if:
- Total cholesterol > 7.5 mmol/L, AND/OR
- Personal or family history (first-degree relative) of premature coronary artery disease (a cardiovascular event < 60 years)
In those with a personal or family history of premature coronary artery disease, a serum lipid profile should be offered if not already completed. The Simon Broome criteria or Dutch Lipid Clinic Network criteria should then be used in those with suspected familial hypercholesterolaemia to make a clinical diagnosis (see below). These patients should be referred to a specialist lipid clinic.
Specialist referral
A specialist referral to a lipid clinic should be made regardless of family history if:
- Clinical diagnosis of FH, OR
- Total cholesterol > 9.0 mmol/L, AND/OR
- LDL cholesterol > 6.5 mmol/L, AND/OR
- Non-HDL cholesterol > 7.5 mmol/L OR
- Fasting triglycerides > 10 mmol/L
An urgent specialist review should be arranged for those with a TG concentration > 20 mmol/L in the absence of excess alcohol or poor glycemic control. The finding of significantly elevated cholesterol and/or triglycerides helps to identify patients likely of having underlying primary hyperlipidaemia.
Simon Broome Criteria
These criteria were developed in the 1980s in the UK. It can be used to make a ‘definitive’ or ‘probable’ diagnosis of familial hypercholesterolaemia. These patients should be referred to a specialist lipid clinic.
Definitive criteria
Total cholesterol > 6.7 mmol/L or LDL-C > 4.0 mmol/L in a child < 16 years old, OR total cholesterol > 7.5 mmol/L or LDL-C > 4.9 mmol/L in an adult, WITH
- Tendon xanthomas in patient or 1st/2nd-degree relative, OR
- Evidence of genetic mutation in LDL receptor or defective Apo B-100 or PCSK9 mutation
Probable criteria
Total cholesterol > 6.7 mmol/L or LDL-C > 4.0 mmol/L in a child < 16 years old, OR total cholesterol > 7.5 mmol/L or LDL-C > 4.9 mmol/L in an adult, WITH
- Family history of myocardial infarction (< 50 years 2nd-degree relative or < 60 years in a 1st-degree relative), OR
- Family history of TC > 7.5mmol/L in adult 1st/2nd degree relative, OR > 6.7mmol/L in child/sibling < 16 years
Lifestyle modifications
Patients with hyperlipidaemia should be advised about lifestyle modifications to reduce their cardiovascular risk.
In patients with hyperlipidaemia, a variety of lifestyle interventions should be discussed with patients to reduce their risk of CAD. These include:
- Dietary advice: total fat intake ≤30% with saturated fats ≤7%. Dietary cholesterol <300 mg/day. Increase mono-unsaturated fat intake (e.g. fats based on olive oil or rapeseed oil). Reduce intake of sugar and food products containing refined sugar. Eat at least 5 portions of fruit and vegetables per day
- Physical activity: advise at least 150 minutes of moderate-intensity aerobic activity per week or 75 minutes of vigorous-intensity activity. In addition, advise ≥2 sessions of muscle-strengthening exercises
- Weight management: offer advice and support in weight loss
- Alcohol consumption: offer advice and support on reducing alcohol intake
- Smoking cessation
Anti-lipid therapies
Statins are the most commonly used anti-lipid therapies that inhibit the enzyme HMG-CoA reductase in the liver.
A variety of anti-lipid therapies are available for the treatment of hyperlipidaemia. Of these, statins form the backbone of treatment and are generally used first-line in all patients. In patients with an inadequate response to statins despite maximal dose or an intolerance, additional therapies can be used. Occasionally, patients will need to be referred for more specialist therapies.
Statins
Statins inhibit 3-hydroxy-3-methylglutaryl- coenzyme A (HMG-CoA) reductase. HMG-CoA is an enzyme found in hepatocytes of the liver. It converts HMG-CoA into mevalonic acid, which is a cholesterol precursor.
The reduction in hepatic cholesterol production leads to upregulation of hepatic LDL receptors that reduce circulating levels of LDL in the blood. The enzyme is most active at night leading to the nocturnal administration of statins.
There are different types of statins including:
- Pravastatin
- Simvastatin
- Atorvastatin
- Rosuvastatin
The intensity of statin therapy describes the extent to which it will reduce LDL-C. High-intensity statins will reduce LDL-C by at least 40%. Whether a statin is high-intensity depends on the type and dose. For example, atorvastatin 20 mg PO once daily is a high-intensity treatment, whereas simvastatin 10 mg PO once daily is a low-intensity treatment.
When starting stains patients require baseline investigations and ongoing monitoring:
- Baseline investigations: serum lipid profile, renal profile, thyroid profile, LFTs, CK (if unexplained muscle pain)
- Monitoring: Serum lipids and LFTs
The BNF has an excellent section on advice for monitoring whilst on statin therapy. For more information see our Statins notes.
Fibrates
Fibrates are commonly used in combination with statins where there has been an inadequate fall in serum lipids. The main fibrate is Fenofibrate. Fibrates not only reduce LDL-C but also help to reduce TG, apolipoprotein B and increase HDL-C.
Fibrates have multiple effects on lipid metabolism, which are generally mediated by the activation of peroxisome proliferator-activated receptors (PPARα), which are a family of nuclear hormone receptors. These actions include:
- Reduce triglyceride synthesis in the liver
- Increase removal of LDL particles
- Induce lipoprotein lipase gene transcription
- Stimulate cellular fatty acid uptake
- Increase HDL production by inducing apo-AI and apo-AII gene transcription
Ezetimibe
Ezetimibe is a selective inhibitor of cholesterol absorption that reduce the delivery of cholesterol to the liver. It may be used as monotherapy or as an adjunct to statin therapy.
Bempedoic acid
This is an adenosine triphosphate citrate lyase (ACL) inhibitor. It works by inhibiting the synthesis of cholesterol in the liver, which lowers LDL-C. It May be used as combined therapy with ezetimibe if there has been an inadequate response and statins are contraindicated or not tolerated.
PCSK9 inhibitors
These are a new class of medications that can be used in the treatment of hyperlipidaemia. These are three main options:
- Alirocumab: monoclonal antibody
- Evolocumab: monoclonal antibody
- Inclisiran: small interfering mRNA
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is an important regulator of cholesterol metabolism. Increased activity of this enzyme is associated with higher levels of LDL-C. PCSK9 is produced by hepatocytes and secreted into the plasma. It is able to bind LDL receptors leading to degradation, which reduces LDL clearance. Some genetic mutations in the gene that encodes this protein are a cause of familial hypercholesterolaemia.
There are various indications outlined by NICE for the use of these medications.
Evinacumab
This is a new monoclonal antibody that targets ANGPTL3 and is being developed for use in familial homozygous hypercholesterolaemia.
Primary prevention
Primary prevention refers to lowering serum lipids before the establishment of cardiovascular disease.
Patients with hyperlipidaemia who are at risk of cardiovascular disease based on their calculated QRISK or underlying co-morbidities should be considered for anti-lipid therapy. Statins form the primary treatment option.
Indications
High-intensity statin therapy (atorvastatin 20 mg PO once at night) should be considered in the following groups:
- Ages ≤84 and QRISK ≥10% over the next ten years (if ≥85 years consider comorbidities, frailty, life expectancy)
- Type 2 diabetes mellitus and QRISK ≥10% over the next ten years
- Type 1 diabetes mellitus and additional risk (Age > 40, diabetes > 10 years, other CVD risk factors, established nephropathy): Can be considered in all patients with Type 1 diabetes mellitus
- Patients with CKD (eGFR < 60 mL/min/1.73m2 and/or albuminuria)
It is important to address all modifiable risk factors in these patients (e.g. smoking, alcohol, diet, obesity) and co-morbidities that may be underestimated by the QRISK (e.g. HIV, systemic inflammatory disorders, severe mental illness).
If lifestyle factors are ineffective or inappropriate then statin therapy with atorvastatin 20 mg PO once at night should be advised (this is a high-intensity statin regimen).
Monitoring & targets
Following initiation of a statin, patients should have a full lipid profile repeated at 3 months. The aim is to achieve a reduction in non-HDL cholesterol by > 40% from baseline (targets are higher in familial hyperlipidaemia).
If the fall in non-HDL cholesterol is < 40% then it is important to check adherence, the timing of dose, diet, and lifestyle. The dose of statin can then be increased until the maximum dose every 2-3 months. If the fall is still < 40% then the addition of a fibrate can be considered or a referral to a specialist lipid clinic. When increasing the dose of statin it is important to consider co-morbidities such as CKD that may affect the dose that can be used.
If patients develop intolerance or side effects then a lower dose or alternative statin should be offered. If these continue then an alternative treatment should be considered (e.g. fibrate).
Secondary prevention
Secondary prevention refers to lowering serum lipids in patients with established cardiovascular disease (e.g. following a heart attack).
Patients with established cardiovascular disease or those with new-onset disease (e.g. recent myocardial infarction) should be started on anti-lipid therapy. The primary treatment is statins. It is still vital to identify and address all modifiable risk factors (e.g. alcohol, smoking, obesity).
Indications
High-intensity statin therapy with maximum dose of atorvastatin (80 mg once a night) should be considered for the following groups that indicate established cardiovascular disease:
- Angina
- Myocardial infarction
- Stroke
- TIA
- Symptomatic peripheral vascular disease
Unlike in primary prevention, it is important not to delay statin therapy whilst addressing modifiable risk factors. Lower doses of atorvastatin should be used in patients with CKD and those with a potential drug interaction or high risk of developing side-effects.
Monitoring & targets
Following initiation of a statin, patients should have a full lipid profile repeated at 3 months. The aim is to achieve a reduction in non-HDL cholesterol by > 40% from baseline (targets are higher in familial hyperlipidaemia).
If the fall in non-HDL cholesterol is < 40% then it is important to check adherence, the timing of dose, diet, and lifestyle. The dose of statin can then be increased if started on a dose lower than 80 mg. If the fall is still < 40% and adherence is good, then additional therapies may be opted for after discussion with the patient. These can include:
- Addition of Ezetimibe
- Addition of PCSK9 inhibitor (if meets eligibility criteria)
Last updated: June 2022
Have comments about these notes? Leave us feedback
