Guillain-Barré syndrome
Notes
Overview
Guillain-Barré syndrome is an acute, inflammatory polyneuropathy typically characterised by a progressive, ascending neuropathy resulting in weakness and reduced reflexes.
GBS is a rare neuropathy classically presenting with progressive lower limb weakness. The majority of patients have history of a preceding illness (typically gastroenteritis or flu-like illness).
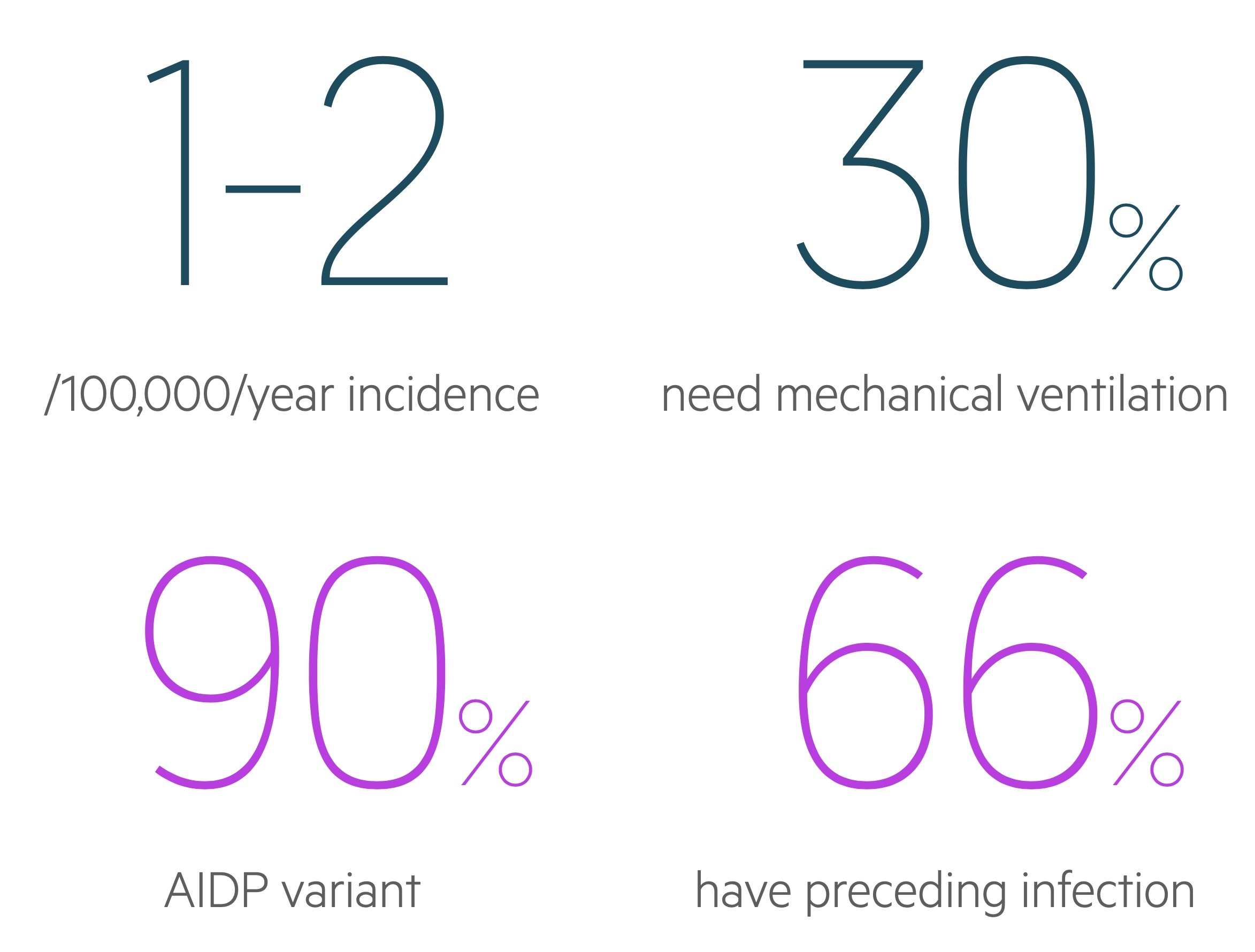
GBS is an umbrella term that refers to a number of ‘variants’ with unique features and pathogenesis. Acute inflammatory demyelinating polyneuropathy (AIDP) is the most common form accounting for 90% of disease.
The condition has a spectrum of severity and may be treated with plasma exchange or IV immunoglobulin. Those with severe disease can require mechanical ventilation.
Epidemiology
The annual incidence is estimated to be 0.6 - 1.9 / 100,000.
As with all rare conditions the epidemiological figures vary widely depending on the study. There appears to be a male predominance. It may occur at any age but is less common at the extremes of age.
Triggers
Campylobacter jejuni is the most common identifiable trigger of GBS.
Campylobacter jejuni
Campylobacter jejuni is a gram-negative rod and common cause of food poisoning and gastroenteritis. In adults it is thought to cause 25-50% of cases.
It appears to be related to more severe GBS when compared to other pathogens with slower recovery and greater risk of permanent disability.
Other infections
Cytomegalovirus, Epstein-Barr virus, hepatitis E and mycoplasma pneumoniae have all been identified as triggers. It may follow a flu-like illness. More recently the Zika virus has been associated with the development of GBS. There appears to be an association in those with HIV.
Pathogenesis & variants
Acute inflammatory demyelinating polyneuropathy is the most common form of GBS.
GBS involves immune-mediated damage to peripheral nerves. One proposed mechanism is ‘molecular mimicry’ where antibodies generated in response to pathogens incorrectly cross-react with the bodies own cells.
The pattern of nerve involvement varies between variants of GBS. The myelin sheath is the target in AIDP (though the axon may be affected) whilst in AMAN and AMSAN axonal degeneration occurs.
Acute inflammatory demyelinating polyneuropathy (AIDP)
The most common form in European countries accounting for an estimated 90% of cases. AIDP presents with ‘classical’ symptoms of GBS:
- Progressive symmetrical weakness in limbs
- Reduced or absent tendon reflexes
- Reduced sensation (may not be clinically apparent to patient)
Symptoms tend to ascend from distal muscles of the limbs and may eventually effect the muscles of respiration. There is commonly a history of preceding viral illness.
AIDP is caused by immune-mediated inflammatory demyelination of peripheral nerves. With time the body may heal these lesions through remyelination over a period of weeks to months. In some patients axonal degeneration occurs resulting in prolonged and incomplete recovery.
Acute motor axonal neuropathy (AMAN)
AMAN is a form of GBS that results from axonal involvement typically following infection with Campylobacter jejuni.
It tends to progress more rapidly than AIDP and does not demonstrate sensory nerve involvement.
Acute motor and sensory axonal neuropathy (AMSAN)
A severe form of AMAN that also demonstrates involvement of sensory nerves. It is characterised by axonal degeneration of both sensory and peripheral nerves.
Miller Fisher syndrome (MFS)
MFS is a variant characterised by a unique presentation:
- Ataxia
- Areflexia
- Ophthalmoplegia
Around 25% will also develop some weakness in the extremities. Up to 90% of patients will have antiganglioside antibodies (anti-GQ1B), a component of nerves.
Investigations
Key investigations in suspected GBS include electromyography, nerve conduction studies and lumbar puncture.
The diagnosis of GBS is suspected based on the clinical presentation. Investigations aim to confirm a diagnosis of GBS and exclude differentials. These include lyme disease, sarcoidosis, thiamine deficiency, porphyria and neoplasms.
Bedside
- Vital signs
- Blood sugar
- Pregnancy test (if indicated)
- Stool culture (if indicated)
- Urine porphobilinogen
Bloods
- FBC
- Renal function
- LFTS
- CRP
- Bone profile & Mg
- HbA1c
- Thyroid profile
- B12/folate/thiamine
- Blood borne virus screen
- Syphilis serology
- Lyme serology
Imaging
CXR: May identify bilateral hilar lymphadenopathy indicative of sarcoidosis (a condition that may cause neuropathies).
MRI spine: Conducted with gadolinium contrast with which enhancement of the caudal equina nerve roots is often seen. Also useful to exclude alternate spinal pathologies.
Special
Electrophysiology: Nerve conduction studies and electromyography can help confirm the diagnosis and differentiate between demyelinating and axonal forms.
Lumbar puncture: Albuminocytologic dissociation - a high CSF protein with normal CSF WCC - is commonly seen but depends on timing from onset of symptoms.
Antibodies: Anti-GQ1b, specific for Miller Fisher syndrome may be sent and is positive in around 90% of cases.
Spirometry: Used to monitor disease progression and involvement of respiratory muscles. Worsening results should trigger anaesthetic/ITU review for consideration of elective intubation.
Management
Plasma exchange or IV immunoglobulin are used to hasten recovery.
Supportive care
Respiratory failure: Patients respiratory function should be regularly monitored with measurements of vital capacity, maximal inspiratory pressure and maximal expiratory pressure. Respiratory failure is not rare as the disease progresses and around 15-30% will need mechanical ventilation as part of their management. Patients with bulbar dysfunction or high aspiration risk may also need early intubation.
Cardiovascular monitoring: Blood pressures may be labile with episodes of both hypo and hyper-tension seen. Both tachy and brady-arrhythmias may occur and telemetry should be considered.
DVT prophylaxis: Unless contraindicated patients should receive lower limb stockings/flowtrons and LMWH prophylaxis.
Analgesia: Neuropathic pain is common and agents such as gabapentin and carbamazepine may be used.
Forced vital capacity
Measuring FVC is an important component of monitoring respiratory function. Measuring FVC should be based on weight and any significant fall should warrant referral to HDU/ITU for closer monitoring, of if significantly low, respiratory support.
Generally, an FVC < 20 ml/kg should warrant referral to HDU/ITU
- If weight 55 kg: FVC < 1.1L, should warrant HDU/ITU referral
- If weight 90 kg: FVC <1.8L, should warrant HDU/ITU referral
Four hourly monitoring is recommended. This should be increased to 1-2 hourly if FVC < 20 ml/kg.
Hughes disability score
The Hughes disability score, also known as the GBS disability score, refers to a functional assessment of patients with GBS. It is graded 0-6 and used in some assessment and treatment pathways.
- 0 - Healthy state
- 1 - Minor symptoms, capable of running
- 2 - Able to walk 10 m or more without assistance but unable to run
- 3 - Able to walk 10 m across an open space with help
- 4 - Bedridden or chairbound
- 5 - Requiring assisted ventilation for at least part of the day
- 6 - Dead
Immunotherapy
There are two main disease modifying treatments for GBS; intravenous immunoglobulin (IVIG) and plasma exchange:
- Intravenous immunoglobulin: Involves the peripheral infusion of immunoglobulin, the mechanism of action is not fully understood. As a blood product there is a risk though low of HIV, Hep B/C or CJD transmission. It is contraindicated in IgA deficient patients where it may cause anaphylaxis.
- Plasma exchange: Patients blood is removed, separated and the plasma removed and replaced. This removes circulating antibodies and other immune modulators.
Both agents have been shown to speed up recovery, combination therapy is not used. Their use is guided by senior neurologists.
Clinical course & prognosis
GBS is described as a progressive post-infectious neuropathy that follows a monophasic disease course.
Typically symptoms develop rapidly over two weeks before plateauing, with recovery beginning around week four. Plasma exchange and IVIG have been shown to speed up recovery.
At 6-months around 80-85% of patients can walk unaided. Relapses are uncommon but affect up to 10% of patients with worsening of weakness seen.
Factors associated with a poorer prognosis include:
- Old age
- Preceding campylobacter jejuni infection
- Rapid onset and severe presenting symptoms
- Need for mechanical ventilation
The one-year mortality is estimated at 3-7% with causes including respiratory failure, infection, cardiovascular dysfunction and pulmonary embolism.
Last updated: June 2021
Have comments about these notes? Leave us feedback
